 Main Menu
Main Menu
|
 Change Themes
Change Themes
|
|
|

12-19-2013, 05:52 PM
|
 |
Member
|
|
Join Date: Jul 2007
Location: Ottawa
Posts: 3,272
|
|
 TM balling webinar synopsis
TM balling webinar synopsis
Many thanks to those that joined our webinar.
One of the highly important topics that came up was the question of imbalances and as the slide show was clear to show, once explained it makes so much sense. I will try my best to put some of that info here.
If anyone highlights an inaccuracy to what I have written please let me know and I will happily address that in the thread openly.
Hearing about the relationship between the ions of Calcium chloride and sodium Bi-carbonate was fascinating especially how the coral polyp takes in the calcium ion from the calcium chloride element and the carbonate ion from the sodium element and what is left behind is your imbalance.
Left behind is sodium from the Bi-carbonate and chloride from the calcium, these two together of course make sodium chloride, and here lies the imbalance in 3 part, all of a sudden you have extra sodium chloride with no other elements attached to it floating around in your tank, and by doing a water change you are only removing the percentage of that water change of the imbalance.
So if you are dosing 2 or 3 part light systems and rely on water changes alone to address the imbalance you are only removing for example on a 10% water change, 10% of that imbalance.
Now - by adding into the mix Part C the remaining sodium chloride has something to balance it which includes the 70 trace elements
Now of course there is an argument that this system too raises your sodium chloride level, and yes you are right, BUT and here is the defining factor, it is doing it in a balanced format in the same way you would be doing by adding more sea salt to your system, because it is balanced there is no ionic risks, and even the most minimal water change would cater for any salinity rise, however due to being in balance and such a very low level this is not an issue, where as an unbalanced system with just sodium chloride floating about is.
What is an issue however are 3 part or light systems that allow for free amounts of sodium chloride in your system with nothing to balance it allowing for a complete imbalance that can not be addressed wholly by water changes as you only remove the % of water change and as such only remove that % of imbalance.
There is only one way to keep a system in balance when dosing calcium and sodium Bi-carbonate and that is to add in proportion NACL free salt. (Part C)
To make the point clearer, the very first original Sea Salt mix from Tropic marin that still to this day forms the basis of all their salts is a 100% mixture of A B and C of the Tropic Marin Balling system together!
So there is no argument chemistry in itself proves it, if you dose a system with nothing to balance the excess NACL you create an imbalance, and this is where part C comes in which is made up of everythign you find in a sea salt mix including all trace elements without adding additional NACL, hence the term for part C as NACL-FREE sea salt. But lets be clear Part C is not just magnesium as in every other other 3 part system, it is the whole bells and whistles found in sea salt as stated before WITHOUT any NACL component.
This is why Tropic marin balling from the inventor Hans-werner balling is so popular to those that care about doing this 100% right.
|
|
|


|

12-20-2013, 11:21 AM
|
|
|
Member
|
Quote:
Originally Posted by Madreefer
I can't believe we've managed to keep our tanks alive for so long without this stuff. I'm pretty confident using the cheap salt as you call it.
|
Thats great as I said before if thats the way you want to go then go for it, but at least add the balancing factor of part C, then you do not have two risks, just one.
But i still do not see the reason of using off the self salts and there are risks involved in them, I have seen many tanks with sudden algal blooms after a year or less, once the person stopped using the salt the issue slowly went away. As a hobbyist you have absolute no control what these salts have in them, you are buying salts not designed for your tank, food grade is great for food, but corals are a little more sensitive, I am not talking about poisons just wrong elements, too much bromide is one possible scenario.
So in "my" view I would rather spend that little extra and get salts from a known pure source where they have been tested and assured for you for the intended use. I also prefer to feed my expensive corals something that I cna trust in as replacing that coral will far outweigh the cost of using a proper salt mix.
But this is where the arguments start so i will end by saying, if you are happy using your DIY salts, go for it, just please at least see the importance of adding Part C. |
|
|
|
 |

12-20-2013, 11:28 AM
|
|
|
Member
|
Quote:
Originally Posted by mrhasan
Well instead of using randy's receipe, if someone just create the mixes according to the doc you posted, they should be getting something closer to what TM's balling is; maybe not 100% accurate but hey, some sacrifices have to be made  I wouldn't mind trying part C since it kind of makes sense now  I would consider it more as "adding traces with one powder" instead of all the ionic balances. Fancy words make things look crazy  |
Yep you are right it is adding all the traces in one powder, but its that one powder with all the traces that enables the ionic balance, it is important to be clear on that point.
I am not so sure about using another salt and copying the recipe, there is a chance you will get a different strength mix as not all salts are made up of equal components, so make sure you at the very least have the correct saturation points as marked, which for the average bobbyist could be difficult to achieve. Which brings me back to the point of why bother when the kit is readily available for you in the first place.
Is there that much need to try and find a way round a product just because it is a commercially supplied product for you, for your ease of use? After you have gone to all these efforts, in effect to beat what many feel as beating the system, or doing it cheaper hoping to get the same results, I am quite sure the saving based on time and effort would be minimal, and in some cases more expensive.
But that's consumer choice, do what you feel fits best with you. Just - yep - PART C at the very least. |
|
|
|
 |

12-20-2013, 02:40 PM
|
|
|
Member
|
Quote:
Originally Posted by mrhasan
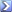
Hows the dosing done Michael? Because to have one sodium ion for every chloride ion, there has to be a ratio of 110.98g of CaCl for every 84.007g of CaHCO (molar masses). Does that satisfy the aprox 10ppm of calcium consumption every 2dkh of alk drop? Because if that amount of grams are not maintained, there will either more be more sodium ions or more chloride ions with an end result of being imbalance.
|
And here is your answer in probably more detail than you could ever wish for  Hans-Werner does not hold back 
• Sodium chloride has a molar weight of 58.44 g/mol.
• In 2 l of R/O water a max. of 2 mol sodium bicarbonate can be dissolved. After addition of 2 mol sodium bicarbonate and 1 mol calcium chloride 2 mol sodium chloride remain in the aquarium. 2 mol sodium chloride can be balanced with exactly 50 g of sodium chloride free sea salt.
• Formula:
CaCl2 x 2 H2O + 2 NaHCO3
CaCO3 + 2 NaCl + CO2 + 3 H2O
• Insert weights:
147 g CaCl2 x 2 H2O + 168 g NaHCO3 100 g CaCO3 + 117 g NaCl + 44 g CO2 + 54 g H2O
117 g NaCl + 50 g NaCl free sea salt 167 g complete sea salt
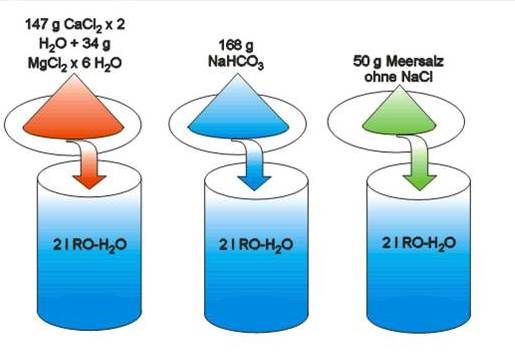
• Adjust tank water to 7° KH and 420 ppm calcium.
• Check alkalinity after two days. Calculate how much alkalinity solution is needed. Add same volume of all three solutions.
• Continue with daily additions of half the volume.
• Adjust added volumes to keep 7° KH alkalinity.
 |
|
|
|
 |

12-21-2013, 01:32 PM
|
|
|
Member
|
Quote:
Originally Posted by Aqua-Digital
...
So in "my" view I would rather spend that little extra and get salts from a known pure source where they have been tested and assured for you for the intended use. I also prefer to feed my expensive corals something that I can trust in as replacing that coral will far outweigh the cost of using a proper salt mix....
|
+1
That's what does it for me. Situations like the bromide issue in Dow Flake from a number of years ago is what concerns me.
You may be able to find less expensive sources of additives, but you have to be a lot more diligent as to keeping up on their composition....IF that information is even available to the public in a timely manner. |
|
|
|
 |

12-30-2013, 05:26 AM
|
|
|
Islander
|
Just mixed the part A , And part B solutions .
Had one issue .
With the part B.
I added 18 scoops to my jug added the water and began shaking. Well no matter what it would not all disolve. So I have that solution now that i dont know what to do with.
So take 2.
This time I weighed each scoop. It only took 15 scoops to reach the 318 grams. This would explain why the first solution would not totally disolve.
anyone else weigh each scoop ? would be nice to know .
really wish i had weighed the other parts first before i hooked up to my doser
|
|
|
|
 |

12-30-2013, 11:46 AM
|
|
|
Member
|
On the box it tells you either scoop or total weight, it makes a lot more sense to use the total weight than a scoop.
As per the instructions
Part A = 380 grams to 5L of RO
Part B = 420g to 5L of RO
Part C = 120g to 5L of RO
scoops are just a guide for those that dont have scales handy, it will never be as accurate as measured weight.
|
|
|
|
 |

12-31-2013, 08:34 PM
|
|
|
Member
|
Glad I read this post before I go ahead and purchase a Calcium reactor, I have a 900G setup, what should I be expecting for a start up?
|
|
|
|
 |

12-31-2013, 08:38 PM
|
|
|
Member
|
Hi
do you mean price or level of ease in setting up?
|
|
|
|
 |

12-31-2013, 08:38 PM
|
|
|
Member
|
level of ease  |
|
|
|
 |

12-31-2013, 08:47 PM
|
|
|
Member
|
very easy, for starters you have full control over every element going into your system where as a calcium reactor you have not a lot of control and will require a kalk stirrer also. Plus the co2 bottles.
With the balling system you need 3 x fluid chambers and the GHL doser and thats it.
start by manually adjusting your parameters over 3 days then set your daily dosing to maintain this, check for the first week every other day then after that weekly.
|
|
|
|
 |
 Posting Rules
Posting Rules
|
You may not post new threads
You may not post replies
You may not post attachments
You may not edit your posts
HTML code is Off
|
|
|
|
 Who's Online
Who's Online
|
There are currently 3 members and 5263 guests. Most users ever online was 34,939, 04-30-2025 at 08:13 AM.
300g, Haroldincum, toxic111
|
 Latest Poll
Latest Poll
|
|
Would you be interested in a Fish QT Service?
This poll is closed
|
| Yes |
13 |
36% |
| No |
11 |
31% |
| Maybe (Depending on cost, QT practices, etc.) |
11 |
31% |
| See Results |
1 |
3% |
| Voters: |
36 |
100% |
|
|
|
 Active Threads
Active Threads
|
|

